Background CX-4945 is an ATP-competitive inhibitor of CK2, which is currently being used in clinical trials to treat various cancer types. Chidamide (CHI), a novel histone deacetylase inhibitor (HDACi), is now involved in the salvage treatment of relapsed/refractory acute myeloid leukemia (AML) in a clinical trial. The anti-leukemia effects of either single drug have been reported by inducing cell apoptosis through suppressing multi-oncogenic pathways in human tumor cells. However, whether and how the combination of the two drugs exerts synergistic anti-tumor effects remains undetermined. This study is to investigate the synergistic therapeutic effects of CX-4945 in combination with CHI in AML.
Methods Cell Counting Kit-8 (CCK-8) cell proliferation assay, Annexin V apoptosis assay, and Propidium Iodide cell cycle assay were performed in AML cells in the presence of CX-4945 only, CHI only, CX-4945 + CHI, and vehicle control for 48h. RNA-seq was performed in U937 cells treated with CX-4945 and CHI, respectively; the differentially expressed genes (DEGs) were identified by R studio, and pathway enrichment of the DEGs was analyzed with Metascape online tool. Data were expressed as the mean ± standard deviation. Ordinary one-way ANOVA statistical analysis and Log-rank test was employed to determine the significant difference between groups. A synergistic effect was determined by the combination indices (CIs) with CalcuSyn software.
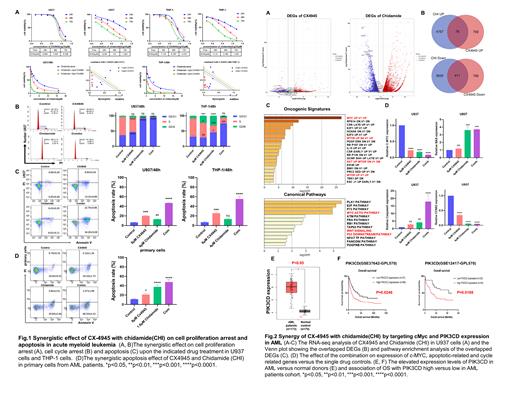
Results The 50% inhibiting concentration (IC50) of CX-4945 on the U937 and THP-1 cell proliferation is 7.435±0.066μmol/L and 6.787±0.153μmol/L. The IC50 of CHI on the U937 and THP-1 cell proliferation is 3.009±0.32μmol/L and 3.782±0.239μmol/L, respectively. The combination of various doses of CHI with either IC50 or 1/2 IC50 of CX-4945 significantly decreases the cell viability of the cells versus single-drug control and CalcuSyn analysis showed the high synergistic therapeutic effects of CHI with CX-4945 (Fig.1A). The combination of (4µM) CHI with (8µM) CX-4945 significantly induced the G0/G1 cell cycle arrest (p <0.001, Fig.1B) and elevated the % of apoptotic cells (p <0.0001, Fig.1C) in the AML cells compared to either single drug alone. Moreover, the combination of (1.6µM) CHI with (8µM) CX-4945 significantly increased the % of apoptotic cells in the primary blasts from the AML patient versus either single drug alone (p<0.0001, Fig.1D). To further understand the underlying mechanism of the synergy, RNA-seq analysis was performed in U937 cells treated with either CHI or CX-4945, respectively (Fig.2A). The DEGs are achieved upon either drug treatment, and the 76 up-regulated and 411 down-regulated overlapped DEGs were identified (Fig.2B). Pathways analysis showed that the overlapped DEGs are enriched in cell cycle progress, apoptosis, and multiple oncogenic signaling including cMyc and PI3K/AKT signaling pathways (Fig.2C). cMyc and PI3K/AKT-dependent signaling pathways are involved in myeloid cell proliferation. Also, our ChIP-seq data indicated obvious Ikaros binding peaks in the promoter region of cMyc and PIK3CD and they are the direct targets of Ikaros (data not shown). cMyc and PIK3CD are at the top of the overlapped DEGs, indicating the critical roles of the cMyc and PIK3CD underlying the mechanism of the combination in AML. Indeed, the mRNA level of cMyc and PIK3CD is significantly down-regulated upon the combination treatment versus single drug control (Fig.2D or data not shown). Consistently, the mRNA level of PIK3CD is significantly higher expressed in AML patients versus that in bone marrow samples of the healthy donors (p<0.05, Fig.2E) and the AML patients with high PIK3CD high expression have shorter OS than that with low expression (p <0.05, Fig.2F). These data support the hypothesis that the combination exerts the anti-tumor effect by suppression of cMyc and PIK3CD expression in AML.
Conclusions The combination of CHI and CX-4945 has a synergistic effect on cell proliferation arrest, apoptosis, and cell cycle arrest by suppressing cMyc and PI3K signaling in AML cells. Our data provide experimental evidence for the combination as a potential option for AML therapy. More pre-clinical studies will be explored for future clinical trials.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal